Hybrid Orbitals
Introduction
Hybrid orbitals are atomic orbitals that result from mixing atomic orbitals in a way that creates new hybrid orbitals. Hybridization occurs when the atomic orbitals of an atom mix to form new orbitals with different shapes and energies.
Hybridization is important because it allows us to understand the bonding and shape of molecules. In this study guide, we will explore the concept of hybridization, pi and sigma bonds, and learn how to predict the hybridization of an atom.
Types of Hybrid Orbitals
There are three types of hybrid orbitals: sp, sp2, and sp3. These hybrid orbitals result from mixing s and p orbitals.
Sp Hybrid Orbitals
Sp hybrid orbitals are formed by mixing one s and one p orbital. This results in two hybrid orbitals that are oriented at a 180-degree angle to each other. These orbitals are linear in shape.
Sp2 Hybrid Orbitals
Sp2 hybrid orbitals are formed by mixing one s and two p orbitals. This results in three hybrid orbitals that are oriented at a 120-degree angle to each other. These orbitals are trigonal planar in shape.
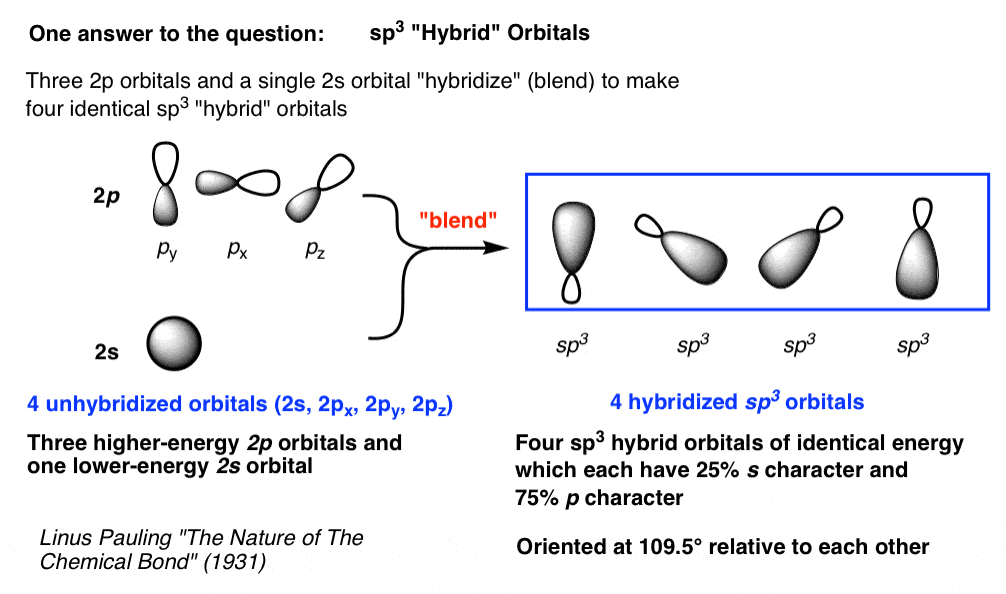
Sp3 Hybrid Orbitals
Sp3 hybrid orbitals are formed by mixing one s and three p orbitals. This results in four hybrid orbitals that are oriented at a 109.5-degree angle to each other. These orbitals are tetrahedral in shape.
Sigma and Pi Bonds
When two atoms form a covalent bond, they share electrons. The electrons are shared in two ways: through a sigma bond and a pi bond.

Sigma Bonds
Sigma (σ) bonds are formed by the end-to-end overlapping of two atomic orbitals. These orbitals can be s or p orbitals. The overlapping region is cylindrically symmetrical around the bond axis. Sigma bonds are strong and provide the primary structural integrity of the molecule. They are typically found in single bonds, such as C-C, C-H, and C-N bonds.
Pi Bonds
Pi (π) bonds are formed by the lateral overlap of two parallel p orbitals. The overlapping region is above and below the bond axis, creating a cloud-like shape. Pi bonds are weaker than sigma bonds and are typically found in double and triple bonds, such as C=C and C≡N bonds. Pi bonds are also found in aromatic compounds, such as benzene.
Predicting Hybridization
To predict the hybridization of an atom, we need to first identify the number of electron groups around the central atom. An electron group is a lone pair or a bond.
Once we know the number of electron groups, we can determine the hybridization using the following guidelines:
- One electron group: sp hybridization
- Two electron groups: sp hybridization
- Three electron groups: sp2 hybridization
- Four electron groups: sp3 hybridization
Conclusion
Hybridization is an important concept in understanding the bonding and shape of molecules. By understanding the types of hybrid orbitals, pi and sigma bonds, and how to predict hybridization, we can better understand the properties of molecules.