Electromagnetic Spectrum and Line Spectra
Electromagnetic Spectrum
The electromagnetic spectrum is the range of all types of electromagnetic radiation. It includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
Equations
Wavelength to frequency
The frequency of an electromagnetic wave is directly proportional to its wavelength, and inversely proportional to the speed of light.
Where:
- is the frequency of the wave, measured in Hertz (Hz)
- is the speed of light, measured in meters per second (m/s)
- is the wavelength of the wave, measured in meters (m)
Wavelength to energy
The energy of an electromagnetic wave is directly proportional to its frequency, and inversely proportional to its wavelength.
Where:
- is the frequency of the wave, measured in Hertz (Hz)
- is the energy of the wave, measured in joules (J)
- is Planck’s constant, measured in joule-seconds (J·s)
Constants
Speed of light
The speed of light is a fundamental constant in physics, and is defined as the speed at which electromagnetic waves propagate in a vacuum.
Planck’s constant
Planck’s constant is a fundamental constant in quantum mechanics, and relates the energy of a photon to its frequency.
Line Spectra
Role of Elections and Photons
- Electrons can be found in different energy levels within an atom.
- When an electron jumps from a higher energy level to a lower one, it releases energy in the form of a photon.
- The electron jumps up to a higher energy level, when when absorbs energy in the form of a photon.
- The energy of the photon determines its wavelength and color.
- The behavior of electrons and photons is fundamental to understanding line spectra.

Emissions
- Emissions occur when an electron releases energy in the form of a photon.
- The resulting line spectra can be used to identify the elements in a sample.
- The number and spacing of the lines are unique to each element.
Absorption
- Absorption occurs when an electron absorbs energy in the form of a photon.
- The energy can cause the electron to jump to a higher energy level.
- When the electron falls back down, it releases a photon with the same energy and wavelength as the absorbed photon.
Lyman
- The Lyman series is a set of emission lines in the ultraviolet region of the spectrum.
- These lines are caused by electrons transitioning to the n=1 energy level.
- The lines are closely spaced and become more closely spaced as they move towards shorter wavelengths.
Balmer
- The Balmer series is a set of emission lines in the visible and near-ultraviolet regions of the spectrum.
- These lines are caused by electrons transitioning to the n=2 energy level.
- The lines are more widely spaced than the Lyman series.
Paschen
- The Paschen series is a set of emission lines in the infrared region of the spectrum.
- These lines are caused by electrons transitioning to the n=3 energy level.
- The lines are even more widely spaced than the Balmer series.
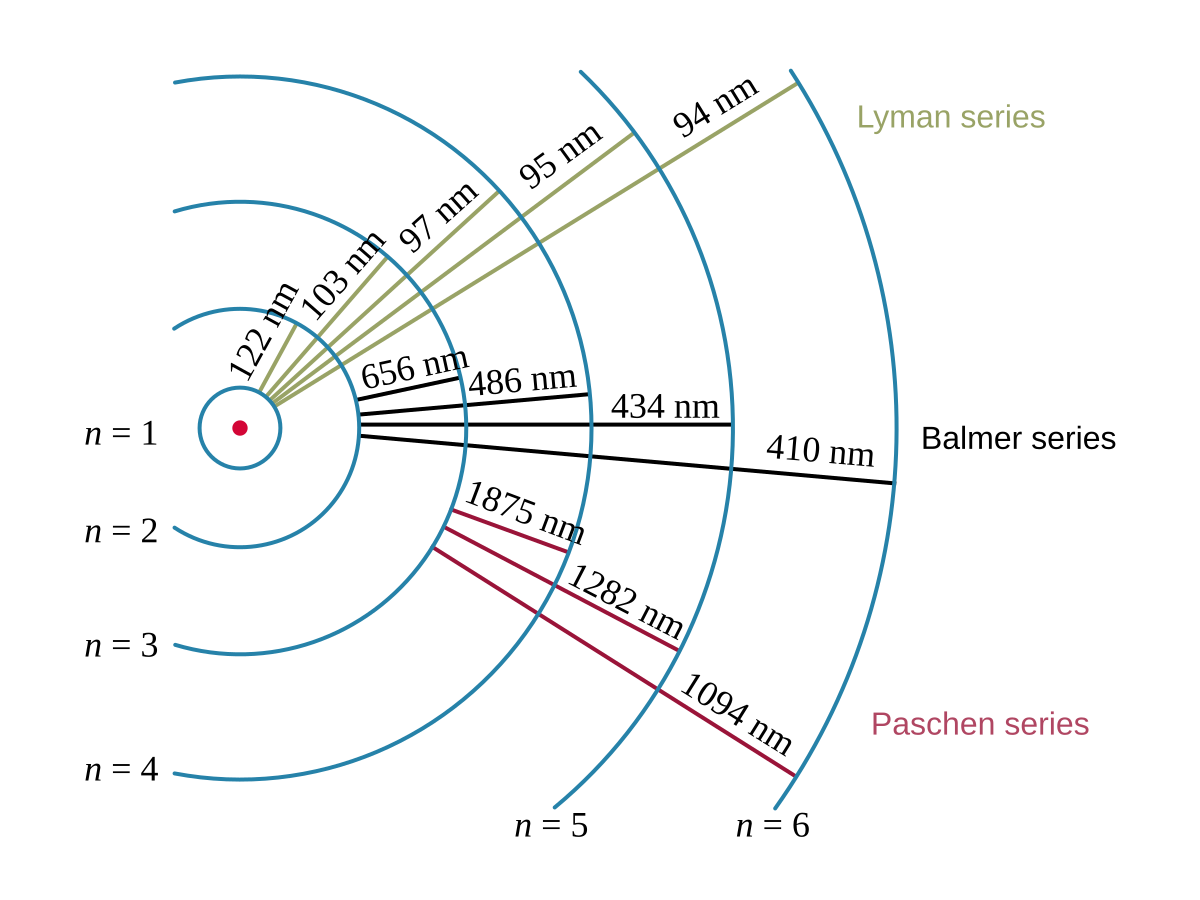
By understanding the behavior of electrons and photons, we can interpret line spectra and identify the elements present in a sample.
Written with StackEdit.